The nitrogen cycle is a biochemical process. Nitrogen is covered in many forms which they will convert into many forms. Like from the atmosphere is converted through the soil to organisms and back to the atmosphere. Nitrogen gas will form in both organic and non-organic forms.
Mostly organic nitrogen exists in living organisms and it passes the food chain through various living organisms. Non-organic forms of nitrogen exist in plants. They make bacteria in the atmosphere which convert nitrites and nitrate. The nitrogen cycle will help the ecosystem in various ways. They maintain their transformation along with the ecosystem. The most complicated biochemical cycle is found in the marine nitrogen cycle.
4 stages of the nitrogen cycle
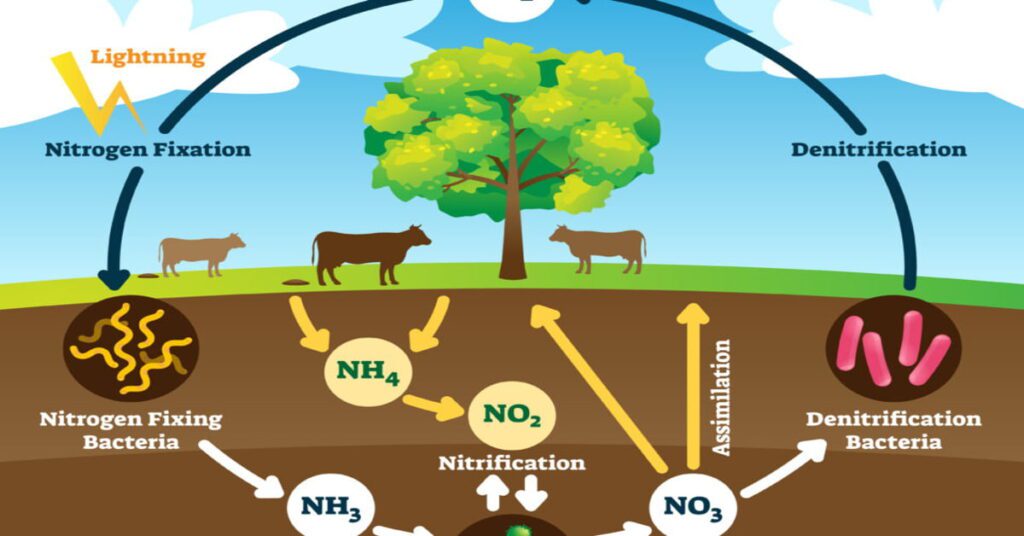

1. Nitrogen fixation in the nitrogen cycle
Nitrogen fixation is a process by which the atmosphere is converted into nitrogen compounds and a biochemical process. The nitrogen fixation molecular formula is N2 which converts air into ammonia. Nitrogen fixation occurs in the soil and aquatic systems. The nitrogen cycle is important for the manufacture of agriculture and fertilizer. N2fixation is mainly focused on the soil by carrying microorganisms.
Some bacteria have a symbiotic relationship with microorganisms in the soil. They will give proper and adequate nutrition and fertilizer to plants. In the rice root, nitrogen fixation is associated with getting micro-organisms and fertilizer in the soil. Plants and animals need nitrogen to produce protein. But they cannot get in the air. Through N2 gas, they create molecules and transform them into soil.
2. Decay and ammonification
Ammonification means the decay of plants and animals. Ammonifying bacteria are found in the death of animals and plants which generate fungi and decay in the soil. To get oxide from nitrates plants and animals require protein which may enter through food items so it is required at each level. Growing a plant step by step produces metabolism and returns to the environment. So accordingly the decay compound is excreted into ammonia.
Ammonification, in chemistry, is defined as the saturation with ammonia or any one of its compounds. Strictly speaking, ammonification refers to any chemical reaction that generates ammonia (NH3) as an end product (or its ionic form, ammonium, NH4+). Ammonification can occur through various inorganic reactions or due to the metabolic functions of microorganisms, plants, and animals. In the ecological context, however, ammonification refers to the processes by which organically bound forms of N2 occurring in dead biomass (such as amino acids and proteins) are oxidized into ammonia and ammonium.
The ecological process of ammonification is carried out in soil and water by a great diversity of microbes and is one of the many types of chemical transformations that occur during the decomposition of dead organic matter.
3. Nitrification
Nitrification is the process in which waste from plants is broken down as ammonium. It is called nitrification. Plants will absorb ammonia and transform it into nitrates in the soil. Also, plants can absorb through their food chain process. Plants are growing due to the decay of materials that convert into nitrates so they can grow faster. Depending on carbon dioxide they store water and get air to grow. Nitrification is present in drinking water either naturally occurring or disinfected to another form. Nitrification will drain the water into the soil to fertilize the plants.
Nitrifying bacteria are chemolithotrophic organisms that include species of genera such as Nitrosomonas, Nitrosococcus, Nitrobacter, Nitrospina, Nitrospira, and Nitrococcus. These bacteria get their energy from the oxidation of inorganic N2 compounds. Types include ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Many species of nitrifying bacteria have complex internal membrane systems that are the location for key enzymes in nitrification: ammonia monooxygenase (which oxidizes ammonia to hydroxylamine), hydroxylamine oxidoreductase (which oxidizes hydroxylamine to nitric oxide – which is further oxidized to nitrite by a currently unidentified enzyme), and nitrite oxidoreductase (which oxidizes nitrite to nitrate).
4. Denitrification of the nitrogen cycle
Denitrification is the process of converting nitrates to N2 gas. It carries out the denitrification of bacteria into the soil. These bacteria live in the depths of the soil and absorb water. It will go deeper and bacteria live longer. During the denitrification process, nitrate in the gaseous form of a large range of microorganisms changes into oxygen in the environment. Fresh water and aquatic water are naturally managed by an ecosystem. fertilizer releases greenhouse gases and tropospheric pollutants.
Unlike nitrification, denitrification is an anaerobic process, occurring mostly in soils and sediments and anoxic zones in lakes and oceans. Similar to nitrogen fixation, denitrification is carried out by a diverse group of prokaryotes, and there is recent evidence that some eukaryotes are also capable of denitrification (Risgaard-Petersen et al. 2006). Some denitrifying bacteria include species in the genera Bacillus, Paracoccus, and Pseudomonas. Denitrifiers are chemoorganotrophs and thus must also be supplied with some form of organic carbon.
Denitrification is important in that it removes fixed nitrogen (i.e., nitrate) from the ecosystem and returns it to the atmosphere in a biologically inert form (N2). This is particularly important in agriculture where the loss of nitrates in fertilizer is detrimental and costly. However, denitrification in wastewater treatment plays a very beneficial role by removing unwanted nitrates from the wastewater effluent, thereby reducing the chances that the water discharged from the treatment plants will cause undesirable consequences (e.g., algal blooms).
Conclusion
The nitrogen cycle is the most important part of the ecosystem and also for humans. The cycle such as N2 fixation, ammonification, nitrification, and denitrification process. These cycles occur because the scarcity of N2 may affect the ecosystem. For the growing of plants and animals, artificial N2 is required to fertilize. N2 is dependent upon organisms for free living. By increasing, the factories and household pollution increase because N2 gas gets mixed.
In terrestrial ecosystems, the addition of nitrogen can lead to nutrient imbalance in trees, changes in forest health, and declines in biodiversity. With increased nitrogen availability there is often a change in carbon storage, thus impacting more processes than just the nitrogen cycle. In agricultural systems, fertilizers are used extensively to increase plant production, but unused nitrogen, usually in the form of nitrate, can leach out of the soil, enter streams and rivers, and ultimately make its way into our drinking water.
The process of making synthetic fertilizers for use in agriculture by causing N2 to react with H2, known as the Haber-Bosch process, has increased significantly over the past several decades. In fact, today, nearly 80% of the nitrogen found in human tissues originates from the Haber-Bosch process (Howarth 2008).
